December 2025
North America News
On 10 November 2025, the United States Environmental Protection Agency released a proposal to make PFAS reporting requirements more practical and implementable, reducing regulatory burden.
The Environmental Protection Agency (EPA) is proposing amendments to the Toxic Substances Control Act (TSCA) regulation related to reporting and recordkeeping requirements for perfluoroalkyl and polyfluoroalkyl substances (PFAS). As outlined October 2023, the regulation requires manufacturers (including importers) of PFAS in any year between 2011-2022 to report certain data to the EPA regarding exposure and environmental and health effects. On 10 November 2025, the EPA proposed incorporating certain exemptions and other modifications to the scope of the reporting regulation. These proposed changes will reduce unnecessary or potentially repetitious reporting requirements for manufacturers.
The proposed exemptions apply to:
PFAS manufactured (including imported) in mixtures or products at concentrations 0.1% or lower
Imported articles
Certain byproducts (chemical substances produced without a separate intent during the manufacture, processing, use, or disposal of another chemical substance or mixture)
Impurities (chemical substances which are unintentionally present with another chemical substance)
Research and development chemicals
Non-isolated intermediates (any intermediate that is not intentionally removed from the equipment in which it is manufactured. An intermediate is any chemical substance that is partially or fully consumed in chemical reactions used intentionally for the manufacture of other chemical substances or mixtures or is intentionally present for altering the rates of such chemical reactions)
Since promulgating the final rule on 11 October 2023, the EPA has moved the reporting deadline twice. In this proposal, further postponement will be needed.
Chapter 173-337 WAC: Safer Products Restrictions and Reporting on PFAS is amended to establish PFAS prohibitions and mandatory reporting obligations for 12 additional consumer product categories, expanding the regulatory scope to encompass 16 categories total.
On 20 November 2025, The Washington State Department of Ecology amended regulation, Chapter 173-337 WAC: Safer Products Restrictions and Reporting on PFAS, which prohibits intentionally added perfluoroalkyl and polyfluoroalkyl (PFAS) and mandates manufacturers to report PFAS in specified product categories. These restrictions and reporting requirements build upon the original regulation enacted in 2023, which addressed intentionally added PFAS in four categories: aftermarket stain- and water-resistant treatments, carpets and rugs, as well as leather and textile furnishings.
Details of the implementation are as follows:
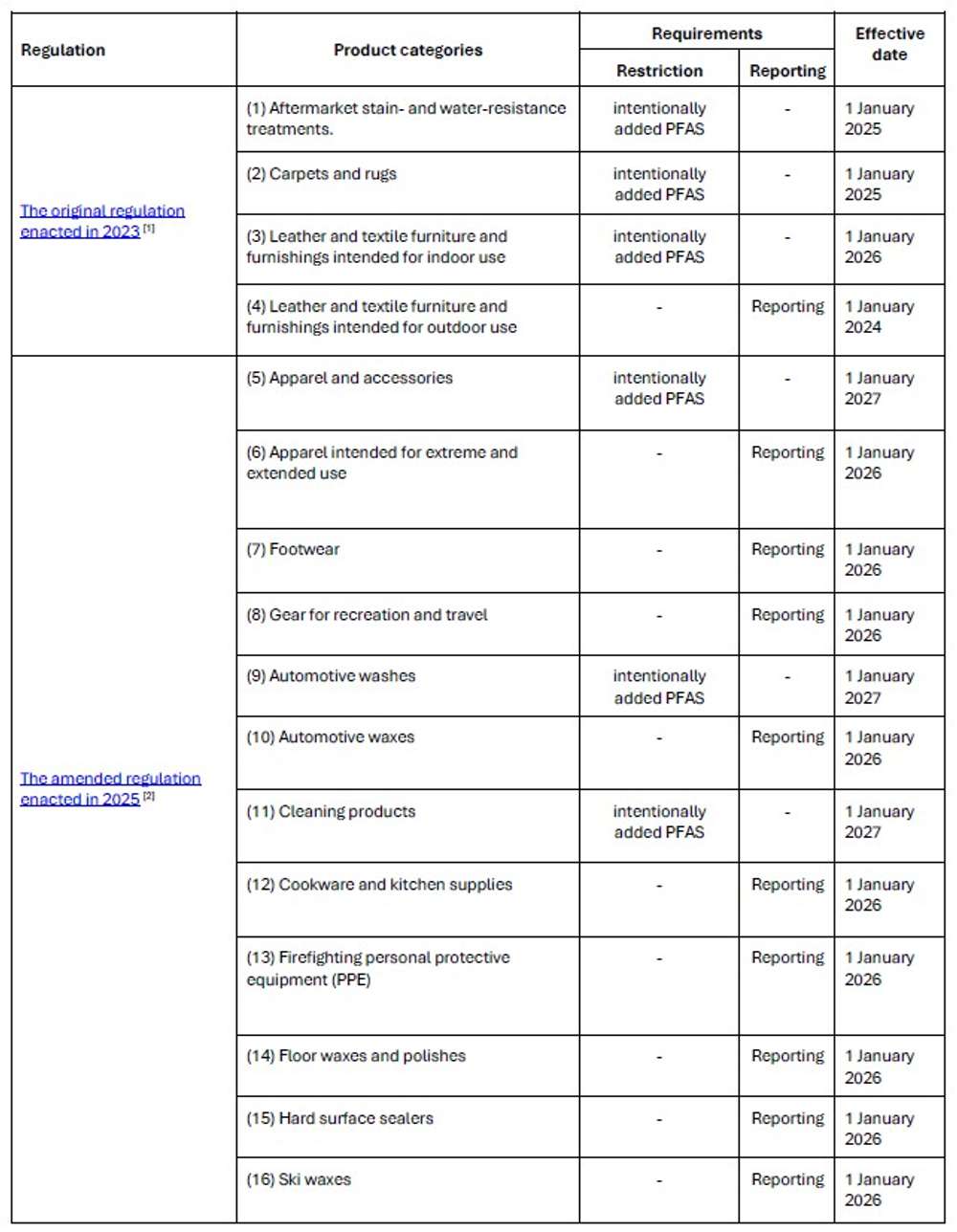
Please note that for the amendments, the reporting period begins on 1 January 2026, with submissions required by 31 January 2027, and recurring annually thereafter. Washington State Department of Ecology presumes the detection of total fluorine above 50 ppm indicates the intentional addition of PFAS; however, manufacturers can rebut this presumption by submitting a statement supported by "credible evidence" that PFAS were not intentionally added to the product.
On 12 December 2025, the CPSC published a final rule to revise 16 CFR 1250, the safety standard for toys. The rule is effective on 12 March 2026. The rule outlines additional performance and labeling requirements for water bead toys and toys that contain water beads.
The US Consumer Product Safety Commission (CPSC) published a final rule on 12 December 2025 related to water beads. The revision to the existing consumer product safety rule for toys, 16 CFR 1250, is to address issues related to the risk of injury or death associated with children mishandling water beads, as well as to address the risks of acrylamide exposure.
The final rule defines a “water bead” as “a various shaped liquid absorbent polymer, composed of materials such as, but not limited to, polyacrylamide and polyacrylate, which expands when soaked in liquid.” By setting a maximum expansion size limit for water bead toys, this rule is intended to reduce the risk of injury or death to children that may result from:
Ingesting water bead(s),
Inserting into their ear or nose,
Aspirating, or
Choking
The rule also is intended to reduce the risks of acrylamide exposure by setting limits on the amount of allowable acrylamide in water bead toys.
Finally, the published rule requires strongly worded, conspicuous warnings.
Based on comments received on the Notice of Proposed Rulemaking (NPR) and clarifications found to be necessary to the rule, the following changes have been made:
The references to “water” in the definition of water bead in proposed section 1250.4(b) have been revised to the broader term “liquid” in the final rule.
In section 1250.4(c)(1) of the final rule, the proposed funnel test gauge diameter has been reduced from 9.0 mm to 5.0 mm; the 50 percent expansion limit has been removed; and an additional test option allowing for the use of a sieve test gauge for testing multiple water beads has been added.
In section 1250.4(c)(2), the extractable acrylamide limit has been changed from 65 μg to 325 μg per 100 small water beads or per 1 large water bead.
In section 1250.4(c)(2), the proposed definitions for small and large water beads describing acrylamide testing, have been changed from “across the smallest diameter” to “in all dimensions” for small water beads, and from “across the smallest diameter” to “in any dimension” for large water beads in the final rule.
In section 1250.4(d), marking, labeling and instructional literature has been updated.
The rule goes into effect on 12 March 2026.
In the US, when hazards are identified in consumer products, they will be recalled and published in the Consumer Product Safety Commission (CPSC) Recent Recalls on the CPSC website, which is updated daily. The US recalls from 01 November 2025 to 30 November 2025 are summarized below:
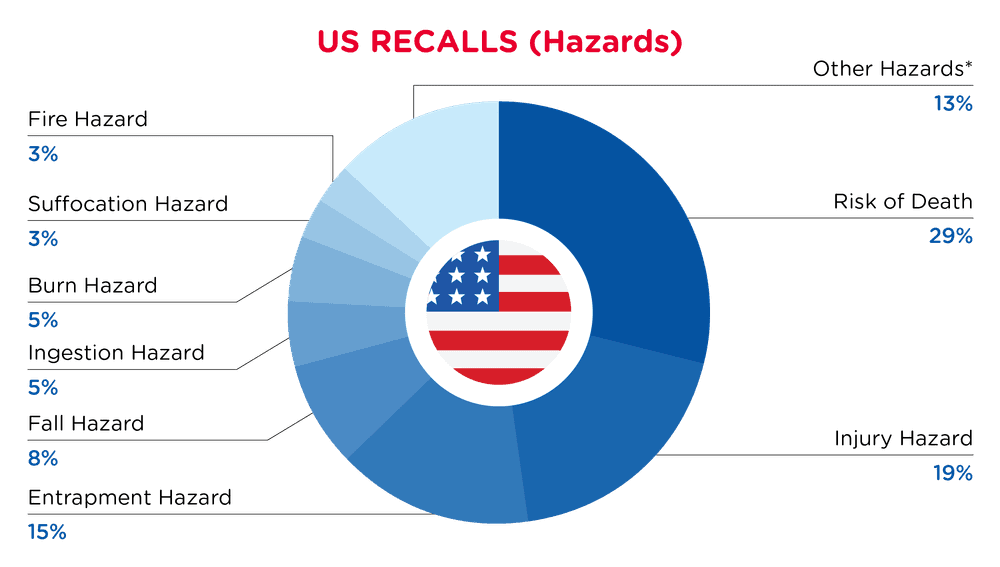
| Hazards | Frequency |
| Risk of Death | 25 |
| Injury Hazard | 17 |
| Entrapment Hazard | 13 |
| Fall Hazard | 7 |
| Ingestion Hazard | 4 |
| Burn Hazard | 4 |
| Suffocation Hazard | 3 |
| Fire Hazard | 3 |
| Other Hazards* | 11 |
*Other Hazards include Aspiration Hazard, Tip-Over Hazard, Laceration Hazard, Choking Hazard, Crash Hazard, Poisoning Hazard and Electric Shock Hazard with a frequency of less than 3.
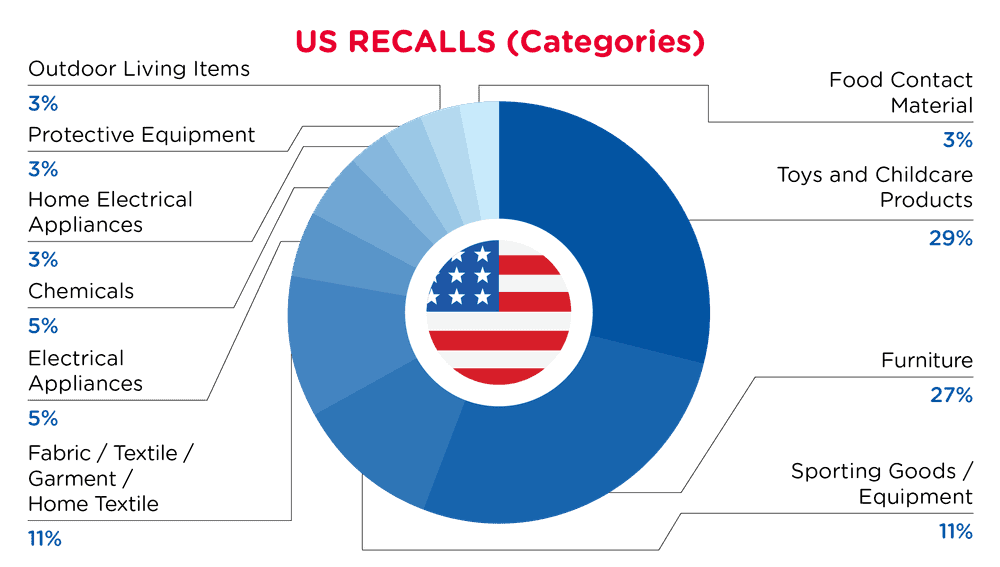
| Product Categories | Frequency |
| Toys and Childcare Products | 11 |
| Furniture | 10 |
| Sporting Goods / Equipment | 4 |
| Fabric / Textile / Garment / Home Textile | 4 |
| Electrical Appliances | 2 |
| Chemicals | 2 |
| Home Electrical Appliances | 1 |
| Protective Equipment | 1 |
| Outdoor Living Items | 1 |
| Food Contact Material | 1 |
For a complete list, click here
In November 2025, Health Canada published updated guidelines interpreting the Children’s Sleepwear Regulations under the Canada Consumer Product Safety Act (CCPSA). The document provides clarity on the scope, definitions, and enforcement approach for children’s sleepwear in sizes up to and including 14X. The update reinforces that items resembling sleepwear—even if marketed for other purposes—remain subject to flammability requirements.
Enforcement measures vary depending on risk and compliance history. The guidelines serve as an unofficial policy document reflecting Health Canada’s current compliance approach.
Health Canada updated its guidelines interpreting the Children’s Sleepwear Regulations under the Canada Consumer Product Safety Act (CCPSA). These new guidelines were published in November 2025 and serve as an unofficial policy document outlining Health Canada’s current approach to compliance. The new guidelines clarify the regulatory scope for garments such as loungewear—which is explicitly considered children’s sleepwear if intended for sleeping or related activities—and outline dimensions used to differentiate children’s from adult sleepwear. For example, chest and seat circumference are used to determine classification, with children’s garments up to size 14X regulated under the Children’s Sleepwear Regulations, while adult garments fall under the Textile Flammability Regulations.
Health Canada confirms that wearable blankets are subject to bedding flammability requirements, with exceptions for blanket sleepers that fall under children’s sleepwear rules. Importantly, the guidelines note that labeling alone cannot exempt garments from the regulation; functional design and marketing will be key in determining classification.
Enforcement actions for non-compliance may include voluntary removal from market, mandatory recall, and prosecution, depending on risk and compliance history. Industry stakeholders are encouraged to consult the guidelines for design and dimensional criteria and to liaise with Health Canada Consumer Product Safety Office for clarifications.
Highlights Table
| Section | Title / Scope | Highlights of Changes / Clarifications |
|---|---|---|
| Introduction & Legislation | Overview of Children’s Sleepwear Regulations | Reinforced applicability to products up to size 14X; applies to Canadian market under CCPSA |
| Definition of Sleepwear | Classification criteria | Clear definition includes loungewear and garments resembling sleepwear regardless of labeling |
| Differentiation from Adult | Measurement guidelines | Use of chest and seat circumference to distinguish children’s vs adult sleepwear |
| Exceptions & Related Items | Wearable blankets and blanket sleepers | Wearable blankets under bedding regs; blanket sleepers under children’s sleepwear regs |
| Enforcement | Compliance expectations | Actions include voluntary removal, mandatory recall, seizure, prosecution based on risk and history |
In Canada, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Health Canada website, which is updated daily. The Canada recalls from 01 November 2025 to 30 November 2025 are summarized below:
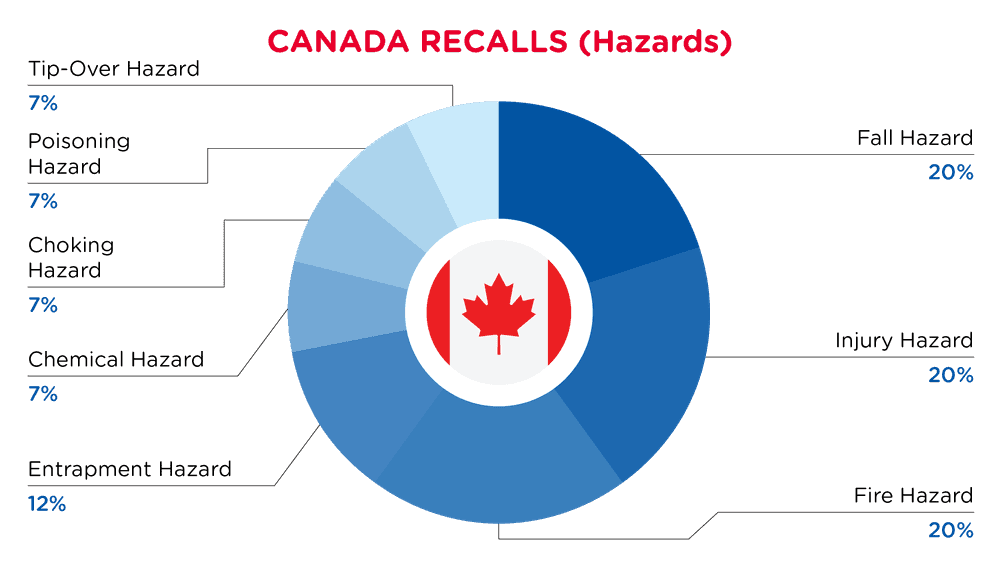
| Hazards | Frequency |
| Fall Hazard | 3 |
| Injury Hazard | 3 |
| Fire Hazard | 3 |
| Entrapment Hazard | 2 |
| Chemical Hazard | 1 |
| Choking Hazard | 1 |
| Poisoning Hazard | 1 |
| Tip-Over Hazard | 1 |
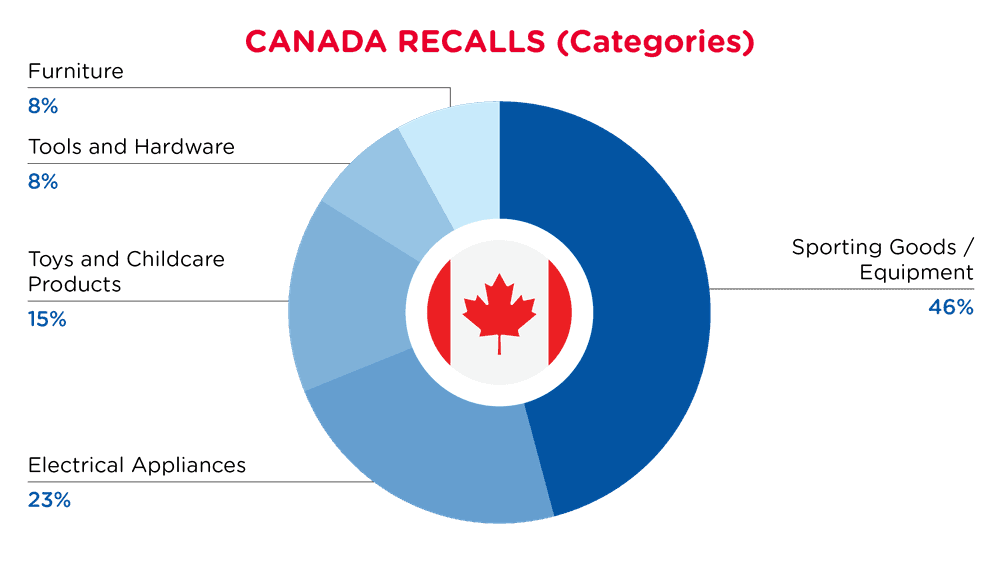
| Product Categories | Frequency |
| Sporting Goods / Equipment | 6 |
| Electrical Appliances | 3 |
| Toys and Childcare Products | 2 |
| Tools and Hardware | 1 |
| Furniture | 1 |
For a complete list, click here
Europe News
In late November 2025, the EU published three amendments for the RoHS directive for the exemption of lead content in various applications.
On 21 November 2025, the European Union (EU) officially announced three amendments for the update to the exemption of the RoHS directive (Restriction of the use of certain Hazardous Substances in electrical and electronic equipment - Directive 2011/65/EU) concerning the use of lead in alloys [Directive (EU) 2025/2364], high-melting-point solders [Directive (EU) 2025/1802], and glass or ceramic components [Directive (EU) 2025/2363].
These amendments shall enter into force 20 days after their official publication (i.e. on 11 December 2025).
These amendments pertain to the exemptions specified in Annex III of Directive 2011/65/EU, including the content in point 6 and point 7. Details of these updates are summarized in the table as below.
Updates for point 6 in Annex III of Directive 2011/65/EU
| Exemption | Scope and dates of applicability | |
|---|---|---|
| 6(a) | Lead as an alloying element in steel for machining purposes and in galvanised steel containing up to 0.35 % lead by weight | Expires on 11 December 2026 |
| 6(a)-I | Lead as an alloying element in steel for machining purposes containing up to 0.35 % lead by weight | Expires on 30 June 2027 for all categories. |
| 6(a)-II | Lead as an alloying element in batch hot-dip galvanised steel components containing up to 0.2 % lead by weight | Expires on 30 June 2027 for all categories. |
| 6(b) | Lead as an alloying element in aluminium containing up to 0.4 % lead by weight | Expires on 11 June 2027 |
| 6(b)-I | Lead as an alloying element in aluminium containing up to 0.4 % lead by weight, provided it stems from lead-bearing aluminium scrap recycling | Expires on 11 December 2026 for categories 1-7, 10. Expires on 30 June 2027 for categories 9 industrial monitoring and control instruments, and 11. |
| 6(b)-II | Lead as an alloying element in aluminium for machining purposes with a lead content up to 0.4 % by weight | Expires on 11 June 2027 for categories 1-7, 10. Expires on 30 June 2027 for categories 9 industrial monitoring and control instruments and 11. |
| 6(b)-III | Lead as an alloying element in aluminium casting alloys containing up to 0.3 % lead by weight provided it stems from lead-bearing aluminium scrap recycling | Expires on 30 June 2027 for categories 1-8, 9 other than industrial monitoring and control instruments, and 10. |
| 6(c) | Copper alloy containing up to 4 % lead by weight | Expires on 30 June 2027. |
Updates for point 7 in Annex III of Directive 2011/65/EU
| Exemption | Scope and dates of applicability | |
|---|---|---|
| 7(a) | Lead in high melting temperature type solders (i.e. lead-based alloys containing 85 % by weight or more lead) | Applies to all categories (except applications covered by point 24 of this Annex) and expires on 30 June 2027. |
| 7(a)-I | Lead in high melting temperature type solders (i.e. lead-based alloys containing 85 % by weight or more lead) for internal interconnections for attaching die, or other components along with a die in semiconductor assembly with steady state or transient/impulse currents of 0.1 A or greater or blocking voltages beyond 10 V, or die edge sizes larger than 0.3 mm × 0.3 mm | Applies to all categories (except applications covered by point 24 of this Annex) and expires on 31 December 2027. |
| 7(a)-II | Lead in high melting temperature type solders (i.e. lead-based alloys containing 85 % by weight or more lead) for integral (meaning internal and external) connections of die attach in electrical and electronic components, if all the following conditions are met: — the thermal conductivity of the cured/sintered die-attach material is > 35 W/(m × K), — the electrical conductivity of the cured/sintered die-attach material is > 4.7 MS/m, — solidus melting temperature is higher than 260 °C | Applies to all categories (except applications covered by point 24 of this Annex) and expires on 31 December 2027. |
| 7(a)-III | Lead in high melting temperature type solders (i.e. lead-based alloys containing 85 % by weight or more lead) in first level solder joints (internal or integral connections – meaning internal and external) for manufacturing components so that subsequent mounting of electronic components onto subassemblies (i.e. modules, sub-circuit boards, substrates, or point-to-point soldering) with a secondary solder does not reflow the first level solder. This sub-entry excludes die attach applications and hermetic sealings | Applies to all categories (except applications covered by point 24 of this Annex) and expires on 31 December 2027. |
| 7(a)-IV | Lead in high melting temperature type solders (i.e. lead-based alloys containing 85 % by weight or more lead) in second level solder joints for the attachment of components to printed circuit board or lead frames: (1) in solder balls for the attachment of ceramic ball-grid-array (BGA); (2) in high temperature plastic overmouldings (> 220 °C) | Applies to all categories (except applications covered by point 24 of this Annex) and expires on 31 December 2027. |
| 7(a)-V | Lead in high melting temperature type solders (i.e. lead-based alloys containing 85 % by weight or more lead) as a hermetic sealing material between: (1) a ceramic package or plug and a metal case; (2) component terminations and an internal sub-part | Applies to all categories (except applications covered by point 24 of this Annex) and expires on 31 December 2027. |
| 7(a)-VI | Lead in high melting temperature type solders (i.e. lead-based alloys containing 85 % by weight or more lead) for establishing electrical connections between lamp components in incandescent reflector lamps for infrared heating, high intensity discharge lamps, or oven lamps | Applies to all categories (except applications covered by point 24 of this Annex) and expires on 31 December 2027. |
| 7(a)-VII | Lead in high melting temperature type solders (i.e. lead-based alloys containing 85 % by weight or more lead) for audio transducers where the peak operating temperature exceeds 200 °C | Applies to all categories (except applications covered by point 24 of this Annex) and expires on 31 December 2027. |
| 7(c)-I | Electrical and electronic components containing lead in a glass or ceramic other than dielectric ceramic in capacitors, e.g. piezoelectronic devices, or in a glass or ceramic matrix compound | Applies to all categories and expires on 30 June 2027. |
Updates for point 7 in Annex III of Directive 2011/65/EU (Cont’d)
| Exemption | Scope and dates of applicability | |
|---|---|---|
| 7(c)-II | Lead in dielectric ceramic in capacitors for a rated voltage of 125 V AC or 250 V DC or higher | Applies to all categories (except applications covered by point 7(c)-I or 7(c)-IV) and expires on 31 December 2027. |
| 7(c)-V | Electrical and electronic components containing lead in a glass or glass matrix compound that fulfils any of the following functions: (1) for protection and electrical insulation in glass beads of high-voltage diodes and glass layers for wafers; (2) for hermetic sealing between ceramic, metal and/or glass parts; (3) for bonding purposes in a process parameter window for < 500 °C combined with a viscosity of 1 013.3 dPas (‘glass-transition temperature’); (4) for use as a resistive material such as ink, with a resistivity range from 1 ohm/square to 100 megohm/square, excluding trimmer potentiometers; (5) for use in chemically modified glass surfaces for microchannel plates (MCPs), channel electron multipliers (CEMs) and resistive glass products (RGPs). | Applies to all categories and expires on 31 December 2027. |
| 7(c)-VI | Electrical and electronic components containing lead in a ceramic that fulfils any of the following functions: (1) for use in piezoelectric lead zirconium titanate (PZT) ceramics; (2) for providing ceramics with a positive temperature coefficient (PTC). | Applies to all categories (except applications covered by points 7(c)-II, 7(c)-III and 7(c)-IV of this Annex as well as point 14 of Annex IV) and expires on 31 December 2027. |
The European Commission has published Notice C (2025) 7699 on 21 November 2025, providing comprehensive guidelines for businesses on the General Product Safety Regulation (EU) 2023/988. This regulation, effective from 13 December 2024, mandates that only safe products be placed on the market in the European Union (EU). The guidelines detail obligations for economic operators and online marketplaces regarding risk assessment, the mandatory EU "Responsible Person," accident reporting, and specific requirements for distance sales.
The (EU) 2023/988 General Product Safety Regulation (GPSR) repeals Directive 2001/95/EC and establishes a general safety requirement for consumer non-food products. European Commission Notice C (2025) 7699 published on 21 November 2025 clarifies the practical implementation of these rules and details the obligations for economic operators and online marketplaces, as outlined below
1. Scope and General Safety Requirement
The GPSR applies to products placed on the market or offered (including online) from 13 December 2024. Products are presumed safe if they comply with relevant European standards referenced in the Official Journal. In the absence of standards, businesses must perform internal risk assessments taking into account the precautionary principle.
2. Economic Operator Obligations
The guidelines distinguish specific duties for different actors. A single business may fall into multiple categories depending on their function for a specific product.
| Actor | Key Obligations |
| Manufacturer | Conduct internal risk analysis. Create technical documentation (retain for 10 years). Ensure product traceability and safety labeling. |
| Importer | Verify manufacturer compliance. Add importer contact details to product/packaging. Ensure storage or transport does not affect product safety and labelling. |
| Distributor | Verify compliance of manufacturer and importer markings. Cooperate with recalls. |
| Responsible Person (EU) | Mandatory for all products. Must hold technical documentation and cooperate with authorities. |
3. Online Marketplaces & Distance Sales
Online Marketplaces: Must register on the Safety Gate Portal, designate a single point of contact for authorities, and process takedown orders within two working days.
Distance Sales Offers: Must visibly display the manufacturer's name/contact, the Responsible Person's details (if manufacturer is non-EU), a product picture/identifier, and warnings.
4. Accident Reporting and Recalls
Businesses must report accidents resulting in death or serious health effects via the Safety Business Gateway. In the event of a recall, consumers must be offered at least two remedies (repair, replacement, or refund), unless it is not possible or is disproportionate. In this case, one remedy should be offered.
Specific to Norway, Medical Devices, Regulation No. 1476, 2021 will be effectively amended, upon implementation of Regulation (EU) 2025/1920) Regulation No. 2423, 2025.
On 2 December 2025, the Norwegian Ministry of Health and Care Services adopted Regulation No. 2423, amending Medical Devices Regulation No. 1476 of 2021.
The Medical Device Ordinance, cf. the Medical Devices Act, Section 1, first paragraph, applies as amended by the following updated regulations, applicable to medical devices:
Regulation (EU) 2020/561
Regulation (EU) 2023/502
Regulation (EU) 2023/607
Regulation (EU) 2023/2197
Regulation (EU) 2025/788
Regulation (EU) 2025/1920
This amendment, referring to Regulation (EU) 2025/1920, updates the Medical Device Regulation (MDR) by introducing specific rules for assigning Master Unique Device Identifiers (UDI-DI) to spectacle frames, spectacle lenses, and ready-to-wear reading spectacles, clarifying what design changes require a new identifier. These provisions will be applicable from 1 November 2028.
The application date is in line with the implemented EU Regulation. This Regulation entered into force on 6 December 2025.
The European Union (EU) adopted the new Toy Safety Regulation (TSR) which will replace Directive 2009/48/EC. The TSR introduces comprehensive updates to chemical, physical, mechanical, and digital safety requirements for toys placed on the EU market, while ensuring alignment with General Product Safety Regulation (EU) 2023/988. A key feature is the introduction of a Digital Product Passport (DPP), which aims to improve transparency, traceability, and market surveillance.
The European Union officially adopted and published the new Toy Safety Regulation (TSR) Regulation (EU) 2025/2509 on 12 December 2025 to strengthen Toy Safety across Europe. This regulation will repeal Directive 2009/48/EC (Toy Safety Directive (TSD)) and aims to address evolving technological, chemical, and consumer safety challenges.
The TSR introduces significant revisions to the TSD, including but not limited to:
1. Scope and Exclusions
The regulation applies to all products designed or intended for play by children under 14 years of age, including products with additional functions beyond play such as digital functions, artificial intelligence (AI) capabilities and connected features
Exclusions from Toy Definition:
Scooters designed for children with a body mass exceeding 20 kg.
Books intended for children older than 36 months, provided they are made entirely of paper or cardboard without play value or added components
2. Enhanced Chemical Safety Requirements
Chemical Safety Assessment Enhancements: Manufacturers must consider both individual chemical exposure and combined exposure hazards in toys
Expansion of Chemical Bans:
| Toy Safety Directive 2009/48/EC | Toy Safety Regulation (EU) 2025/2509 |
|---|---|
| Substances classified as carcinogenic, mutagenic, or toxic for reproduction (CMR substances) under Regulation (EC) No. 1272/2008 | Substances under Regulation (EC) 1272/2008 classified as: • Carcinogenic, mutagenic, or toxic for reproduction (CMR substances) • Endocrine disruptors (ED) categories 1 or 2 for human health and the environment • Specific target organ toxicity (STOT) category 1 (single or repeated exposure) • Respiratory sensitizers category 1 • Skin sensitizers category 1A |
Prohibition of Specific Chemicals:
The intentional use of per- and polyfluoroalkyl substances (PFAS) is prohibited
Thirty-four bisphenols identified by the European Chemicals Agency (ECHA) as toxic for reproduction or endocrine disruptors under the Classification, Labeling and Packaging of chemicals (CLP) regulation 1272/2008 and the following bisphenols are banned in toys:
| CAS Number | Name of Bisphenol |
|---|---|
| 77-40-7 | 4,4'-(1-methylpropylidene)bisphenol (bisphenol B, BPB) |
| 79-97-0 | 4,4’-isopropylidenedi-o-cresol (bisphenol C, BPC) |
| 85-60-9 | 6,6'-di-tert-butyl-4,4'- butylidenedi-m-cresol |
| 118-82-1 | 2,2',6,6'-tetra-tert-butyl-4,4'-methylenediphenol (TBMD) |
| 1745-89-7 | 4,4'-isopropylidenebis[2-allylphenol] |
| 5613-46-7 | 4,4'-isopropylidenedi-2,6-xylol |
| 19224-29-4 | 2,2'-[(1-methylethylidene)bis(4,1-phenyleneoxy)]bisethyl diacetate |
| 27689-12-9 | (1-methylethylidene)bis(4,1-phenyleneoxy-3,1-propanediyl) bismethacrylate |
| 95235-30-6 | 4-(4-isopropoxyphenylsulfonyl)phenol |
| 41481-66-7 | 2,2'-diallyl-4,4'-sulfonyldiphenol (TG-SA) |
Chemical Substance Requirements Updates:
Nitrosamines and nitrosatable substances in toys are restricted in five categories of toys:
| N-nitrosamines (mg/kg) | N-nitrosatable substances (mg/kg) | |
|---|---|---|
| a) Toys intended for use by children under 36 months and intended or likely to be placed in the mouth | 0.01 | 0.1 |
| b) Toys intended for use by children under 36 months not covered by a) above | 0.05 | 1.0 |
| c) Toys intended for use by children of 36 months and over and intended to be placed in the mouth | 0.05 | 1.0 |
| d) Balloons | 0.05 | 1.0 |
| e) Finger paints, slimes and putties | 0.02 | 1.0 |
*new or revised requirements are in italic and blue
Restrictions are imposed on the following chemicals across all toys.
| Substance | CAS Numbers | Limit |
|---|---|---|
| Tris(2-carboxyethyl)phosphine (TCEP) | 115-96-8 | 5 mg/kg |
| Tris(1-chloro-2-propyl)phosphate (TCPP) | 13674-84-5 | 5 mg/kg |
| Tris(1,3-dichloro-2-propyl) phosphate (TDCP) | 13674-87-8 | 5 mg/kg |
| Formamide | 75-12-7 | 20 μg/m3 (emission limit) after a maximum of 28 days from commencement of the emission testing of foam toy materials containing more than 200 mg/kg (cut-off limit based on content) |
| 1,2-benzisothiazol 3(2H)-one (BIT) | 2634-33-5 | 5 mg/kg in aqueous toy materials |
| 5-Chloro-2-methyl isothiazolin-3(2H)-one (CIT) | 26172-55-4 | 0.75 mg/kg in aqueous toy materials |
| Phenol | 108-95-2 | 5 mg/l (migration limit) in polymeric materials 10 mg/kg (content limit) as a preservative |
| Formaldehyde | 50-00-0 | 1.5 mg/l (migration limit) in polymeric toy material 0.062 mg/m3 (emission limit) in wood toy material 30 mg/kg (content limit) in textile toy material 30 mg/kg (content limit) in leather toy material 30 mg/kg (content limit) in paper toy material 10 mg/kg (content limit) in water-based toy material |
| Aniline | 62-53-3 | 30 mg/kg (content limit) after reductive cleavage in textile toy material and leather toy material 10 mg/kg (content limit) as free aniline in finger paints 30 mg/kg (content limit) after reductive cleavage in finger paints |
| Styrene | 100-42-5 | 0.77 mg/l (migration limit) in polymeric toy materials |
| Bisphenol A | 80-05-7 | 0.005 mg/l (migration limit) |
| Acrylonitrile | 107-13-1 | 0.01 mg/l (migration limit) in polymeric toy materials |
| Butadiene | 106-99-0 | 0.07 mg/l (migration limit) in polymeric toy materials |
| Vinyl chloride | 75-01-4 | 0.01 mg/l (migration limit) in polymeric toy materials |
*new or revised requirements are in italic and blue
Allergenic Fragrance Updates:
Allergenic fragrances are prohibited in toys intended for children under 36 months or those meant to be placed in the mouth unless their presence is technically unavoidable under Good Manufacturing Practices (GMP) and do not exceed a concentration of 10 mg/kg (reduced from the current limit of 100 mg/kg)
Fragrances must be listed on the toy's label or accompanying documentation and included in the Digital Product Passport (DPP)
3. Physical and Mechanical Safety Enhancements
Establishes maximum values for both impulse noise and continuous noise emitted by sound producing toys based on scientific studies and recommendation from medical experts
Special attention is given to toys associated with food, which present unique choking hazards distinct from those posed by toys alone
4. Warnings
Safety Pictogram Requirements: Toys unsuitable for children under 36 months must display a pictogram with a minimum diameter of 10 mm
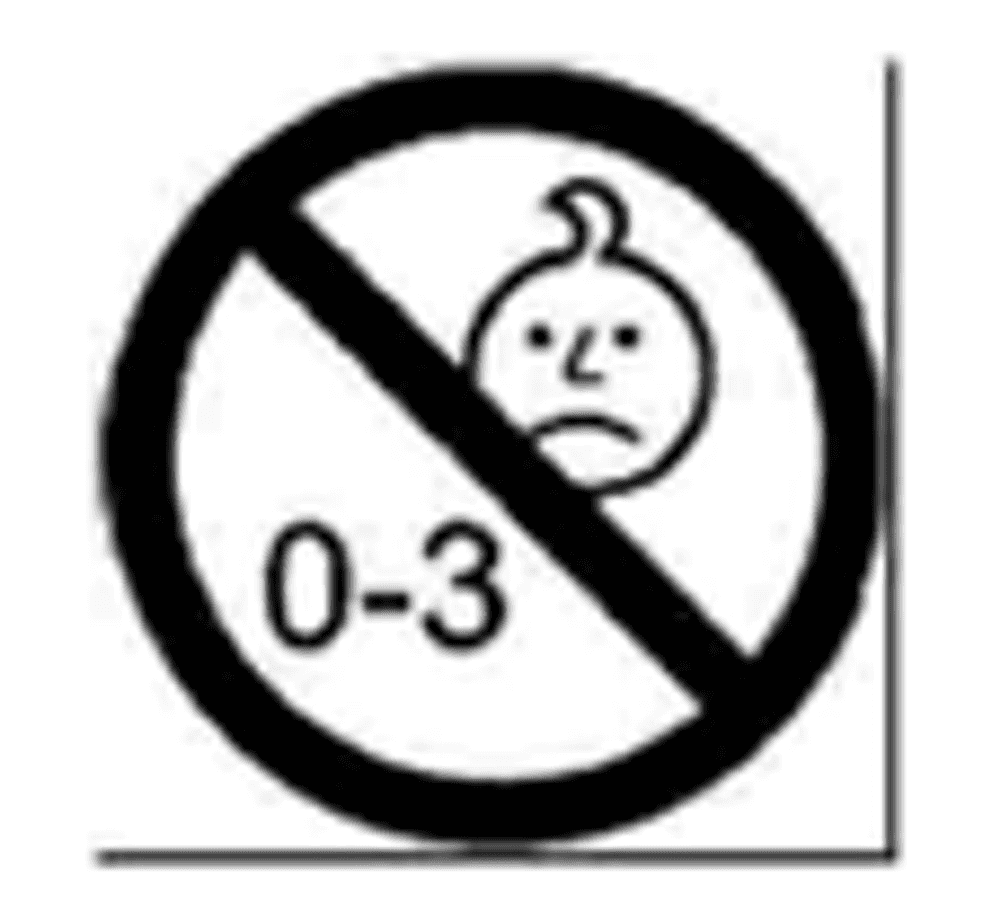
All warnings shall be preceded by the word “Warning” or by a generic pictogram which shall be displayed in a prominent way

5. Digital Product Passport
The DPP will replace the EU declaration of conformity (DoC). It will include product-specific data such as the DoC and additional information accessible electronically via a data carrier
The Commission is required to adopt delegated acts within 12 months after the date of entry into force (EIF) of the TSR to define technical requirements for the DPP
The Commission will issue practical guidelines and tailor-made guidance to assist small and medium-sized enterprises (SMEs) in establishing a DPP. An automatic translation tool must be provided for official languages in Member States where the toy is marketed. These resources will be available within one year after the EIF of the TSR
A back-up copy of the DPP shall be available through a digital product passport service provider
Completing the DPP and applying the CE marking indicates the manufacturer is making a declaration that their toy follows the requirements of the Regulation and takes full responsibility for the toy
6. Integration with the General Product Safety Regulation (GPSR)
The TSR is designed to operate complementary to GPSR (EU) 2023/988. Online marketplaces must adhere to Article 22 of Regulation (EU) 2023/988 on product safety
7. Technical Documentation
Amended elements to be included in the technical documentation:
A detailed description of the design and manufacture including a list of components and materials used in the toy
A list of substances and mixtures used, to include the safety data sheet (SDS), is required to be part of the technical documentation
Transitional Provisions:
The TSR will enter into force on 1 January 2026
Current Directive 2009/48/EC shall be repealed on 1 August 2030 and only
will apply. Clauses including those on conformity assessment will apply from 1 January 2026
On 15 December 2025, the European Commission adopted Commission Implementing Decision (EU) 2025/2519, amending Implementing Decision (EU) 2023/740 to update references to harmonized standards under the Toy Safety Directive (Directive 2009/48/EC). The Decision introduces updated A2 versions of standards relating to the migration of certain elements from toys and to olfactory board games, cosmetic kits and gustative games, and establishes transitional application dates.
To update references to harmonized standards under the Toy Safety Directive (Directive 2009/48/EC), the European Commission adopted Commission Implementing Decision (EU) 2025/2519, amending Implementing Decision (EU) 2023/740 on 15 December 2025.
The Decision updates the list of harmonized toy safety standards published in the Official Journal by:
Adding EN 71-3:2019+A2:2024 (Migration of certain elements); and
Adding EN 71-13:2021+A2:2024 (Olfactory board games, cosmetic kits and gustative games).
Earlier versions of these standards, as itemized below, will be withdrawn from the list beginning 16 June 2027.
EN 71-3:2019+A1:2021 (Migration of certain elements); and
EN 71-13:2021+A1:2022 (Olfactory board games, cosmetic kits and gustative games).
Until this date, both versions of these standards are harmonized and valid. The manufacturer may continue to rely on previously listed versions of the standards for presumption of conformity. After this date, only the updated A2 versions can be used.
In Europe, when hazards are identified in non-food consumer products, the products will be recalled and published in the Safety Gate system, which is updated weekly. The European recalls from 01 November 2025 to 30 November 2025 are summarized below:
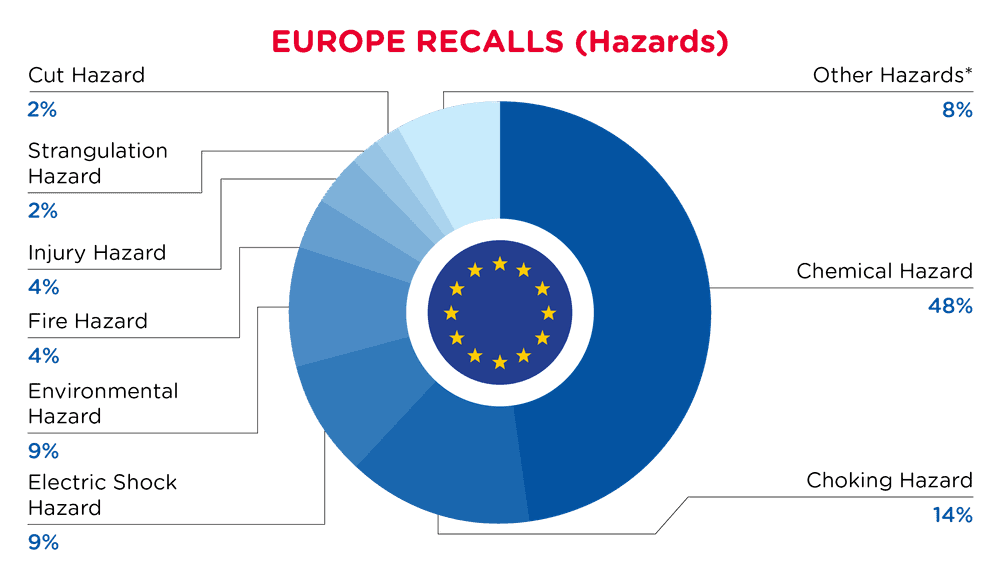
| Hazards | Frequency |
| Chemical Hazard | 192 |
| Choking Hazard | 57 |
| Electric Shock Hazard | 36 |
| Environmental Hazard | 34 |
| Fire Hazard | 16 |
| Injury Hazard | 14 |
| Strangulation Hazard | 10 |
| Cut Hazard | 7 |
| Other Hazards* | 30 |
*Other Hazards include Burn Hazard, Suffocation Hazard, Drowning Hazard, Damage to Hearing, Health Risk Hazard, Microbiological Hazard and Entrapment Hazard with a frequency of less than 7.
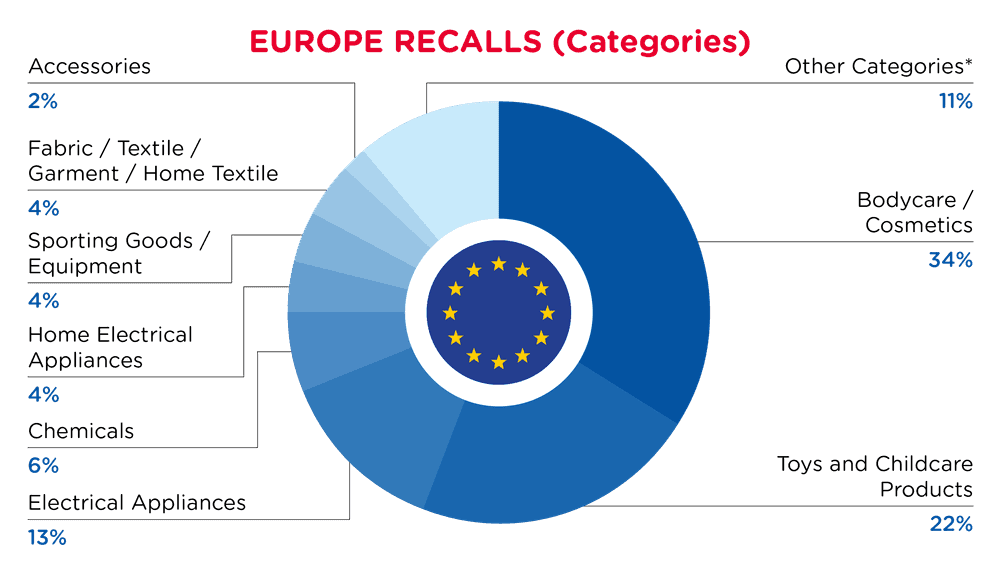
| Product Categories | Frequency |
| Bodycare / Cosmetics | 117 |
| Toys and Childcare Products | 76 |
| Electrical Appliances | 45 |
| Chemicals | 21 |
| Home Electrical Appliances | 15 |
| Sporting Goods / Equipment | 14 |
| Fabric / Textile / Garment / Home Textile | 13 |
| Accessories | 8 |
| Other Categories* | 39 |
*Other Categories include Protective Equipment, Computer / Audio / Video / Other, Electronics & Accessories, Footwear, Jewelry, Furniture, Household Items, Tools and Hardware, Machinery, Food Contact Material, Pet Items and Car Accessories with a frequency of less than 8.

| Notifying Country | Frequency |
| Hungary | 58 |
| Sweden | 42 |
| France | 38 |
| Czechia | 38 |
| United Kingdom in respect of Northern Ireland | 30 |
| Italy | 29 |
| Lithuania | 17 |
| Finland | 15 |
| Germany | 14 |
| Malta | 13 |
| Latvia | 13 |
| Ireland | 12 |
| Other Countries | 29 |
*Other Countries include Poland, Slovakia, Austria, Cyprus, Bulgaria, Slovenia, Spain, Croatia, Norway, Luxembourg and Estonia with a frequency of less than 10.
For a complete list, click here
China News
In China, when hazards are identified in consumer products, they will be recalled and published in the SAMR Defective Product Administrative Centre, which is updated daily. The China recalls from 01 November 2025 to 30 November 2025 are summarized below:
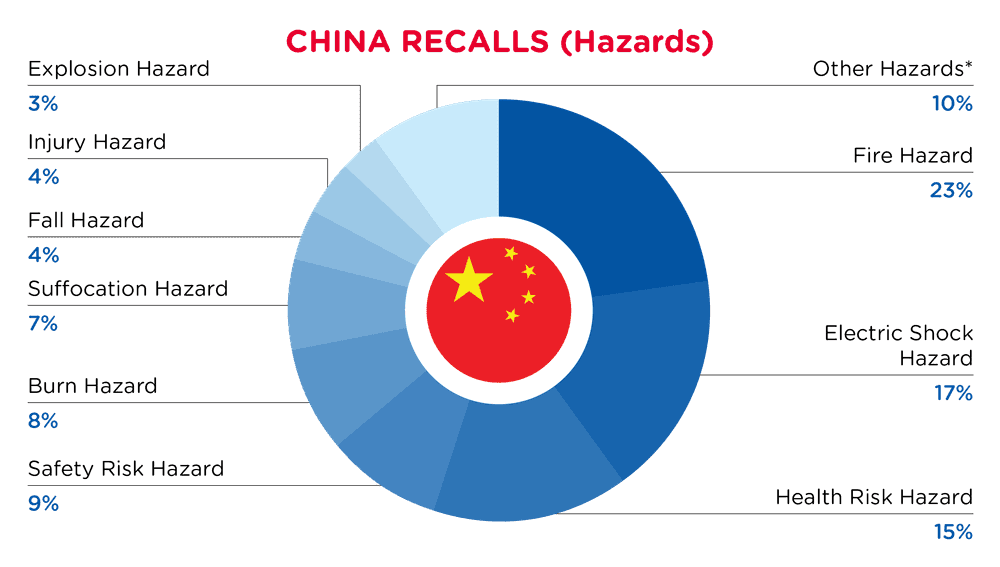
| Hazards | Frequency |
| Fire Hazard | 25 |
| Electric Shock Hazard | 19 |
| Health Risk Hazard | 17 |
| Safety Risk Hazard | 10 |
| Burn Hazard | 9 |
| Suffocation Hazard | 8 |
| Fall Hazard | 4 |
| Injury Hazard | 4 |
| Explosion Hazard | 3 |
| Other Hazards* | 11 |
*Other Hazards include Entanglement Hazard, Damage to Sight, Carbon Monoxide Poisoning Hazard, Tip-Over Hazard, Crash Hazard, Skin Irritation Risk, Swallowing Risk and Laceration Hazard with a frequency of less than 3.
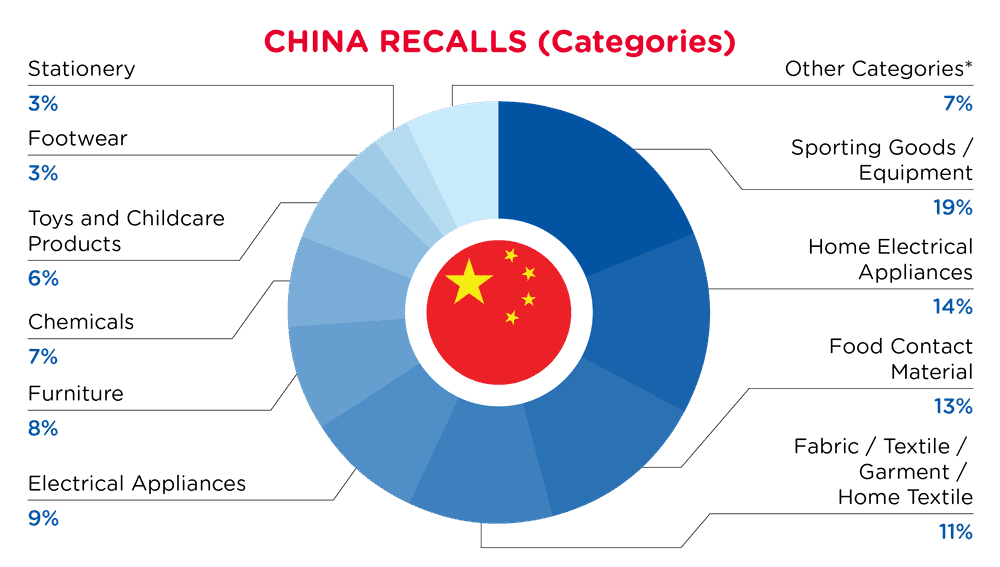
| Product Categories | Frequency |
| Sporting Goods / Equipment | 17 |
| Home Electrical Appliances | 13 |
| Food Contact Material | 12 |
| Fabric / Textile / Garment / Home Textile | 10 |
| Electrical Appliances | 8 |
| Furniture | 7 |
| Chemicals | 6 |
| Toys and Childcare Products | 5 |
| Footwear | 3 |
| Stationery | 3 |
| Other Categories* | 6 |
*Other Categories include Protective Equipment, Accessories, Household Items and Bodycare / Cosmetics with a frequency of less than 3.
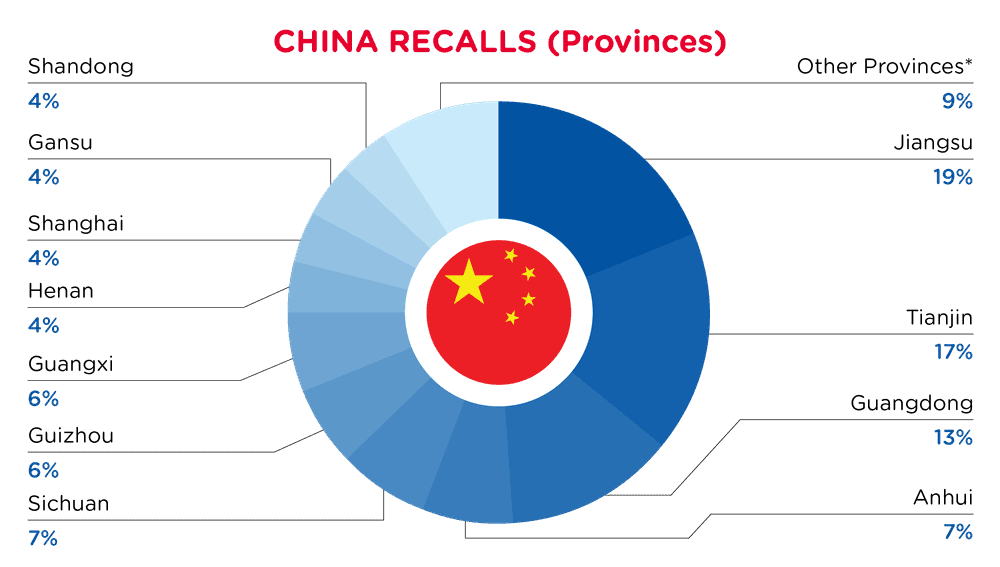
| Provinces | Frequency |
| Jiangsu | 17 |
| Tianjin | 15 |
| Guangdong | 12 |
| Anhui | 6 |
| Sichuan | 6 |
| Guizhou | 5 |
| Guangxi | 5 |
| Henan | 4 |
| Shanghai | 4 |
| Gansu | 4 |
| Shandong | 4 |
| Other Provinces* | 8 |
*Other Provinces include Fujian, Beijing, Hunan and Xinjiang with a frequency of less than 4.
For a complete list, click here
Australia/New Zealand News
In Australia, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Australian Competition & Consumer Commission website, which is updated daily. The Australia recalls from 01 November 2025 to 30 November 2025 are summarized below:
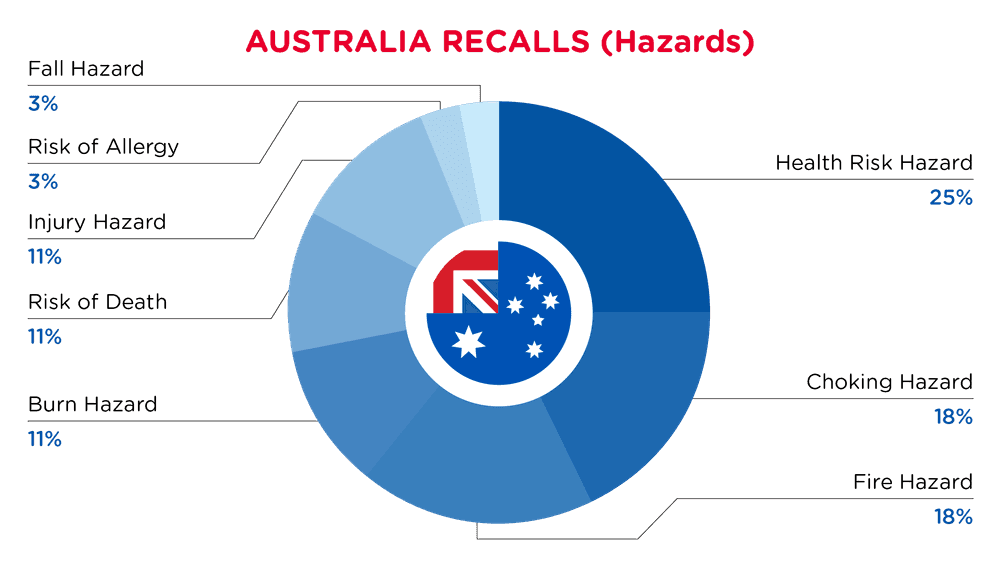
| Hazards | Frequency |
| Health Risk Hazard | 7 |
| Choking Hazard | 5 |
| Fire Hazard | 5 |
| Burn Hazard | 3 |
| Risk of Death | 3 |
| Injury Hazard | 3 |
| Risk of Allergy | 1 |
| Fall Hazard | 1 |
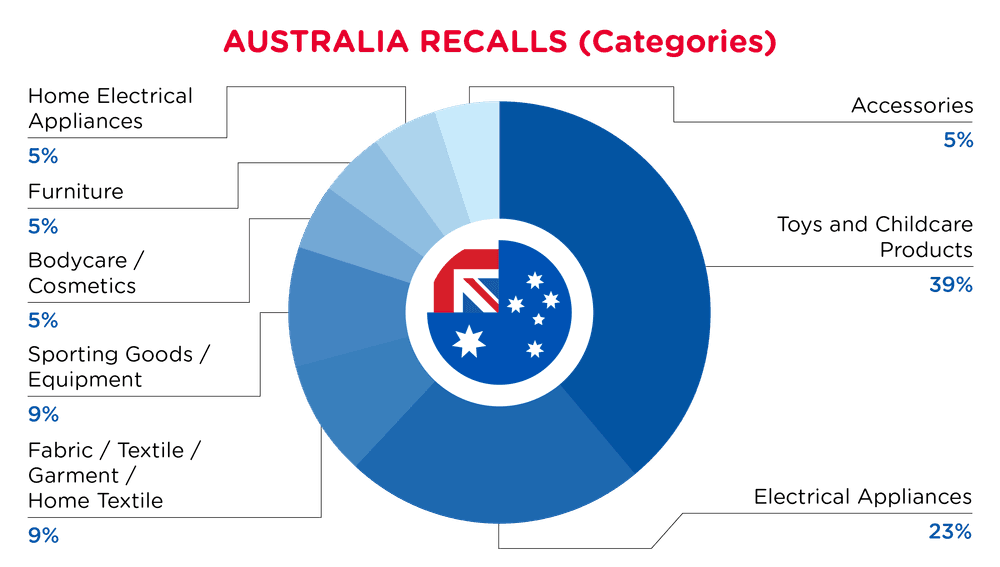
| Product Categories | Frequency |
| Toys and Childcare Products | 9 |
| Electrical Appliances | 5 |
| Fabric / Textile / Garment / Home Textile | 2 |
| Sporting Goods / Equipment | 2 |
| Bodycare / Cosmetics | 1 |
| Furniture | 1 |
| Home Electrical Appliances | 1 |
| Accessories | 1 |
For a complete list, click here
Subscribe to our Regulatory Updates
Unsubscribe at any time. Read our privacy policy.